Credit: NICHD/J. Lippincott-Schwartz
Precision immunotherapy for autoimmune diseases
Curative treatment approaches for autoimmune and rheumatic diseases do not exist. Current treatment strategies for rheumatic disease rely on broadly acting immunosuppressive drugs that reduce self-reactive immune responses but also impair protective immunity, resulting in infection, poor vaccine responses, and treatment-related morbidity. In addition, available therapies often lack desired the potency to control disease and therapeutic responses are not predictable.
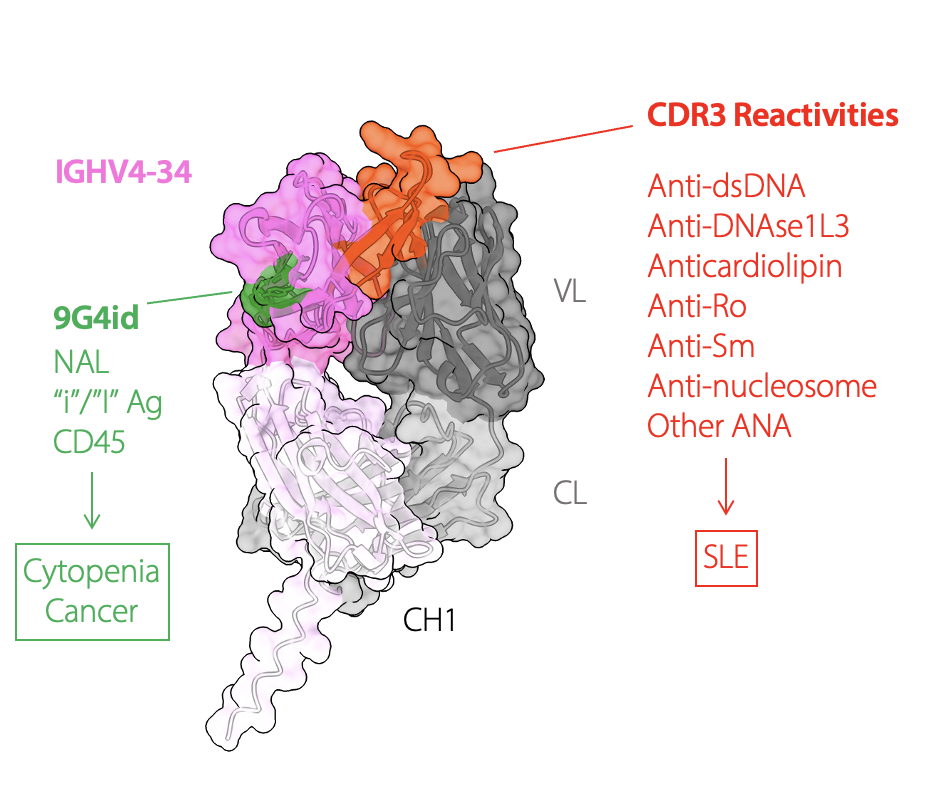
Ideal treatments for autoimmune diseases would eliminate only the self-reactive immune cells that cause harm but preserve normal immune responses. Our goal is to develop personalized and antigen-specific immunotherapies that can achieve that, using orthogonal technologies and engineering platforms developed in our lab.
CAR-T and Precision Cellular Immunotherapies (Nature Rev Rheumatol. 2024.)
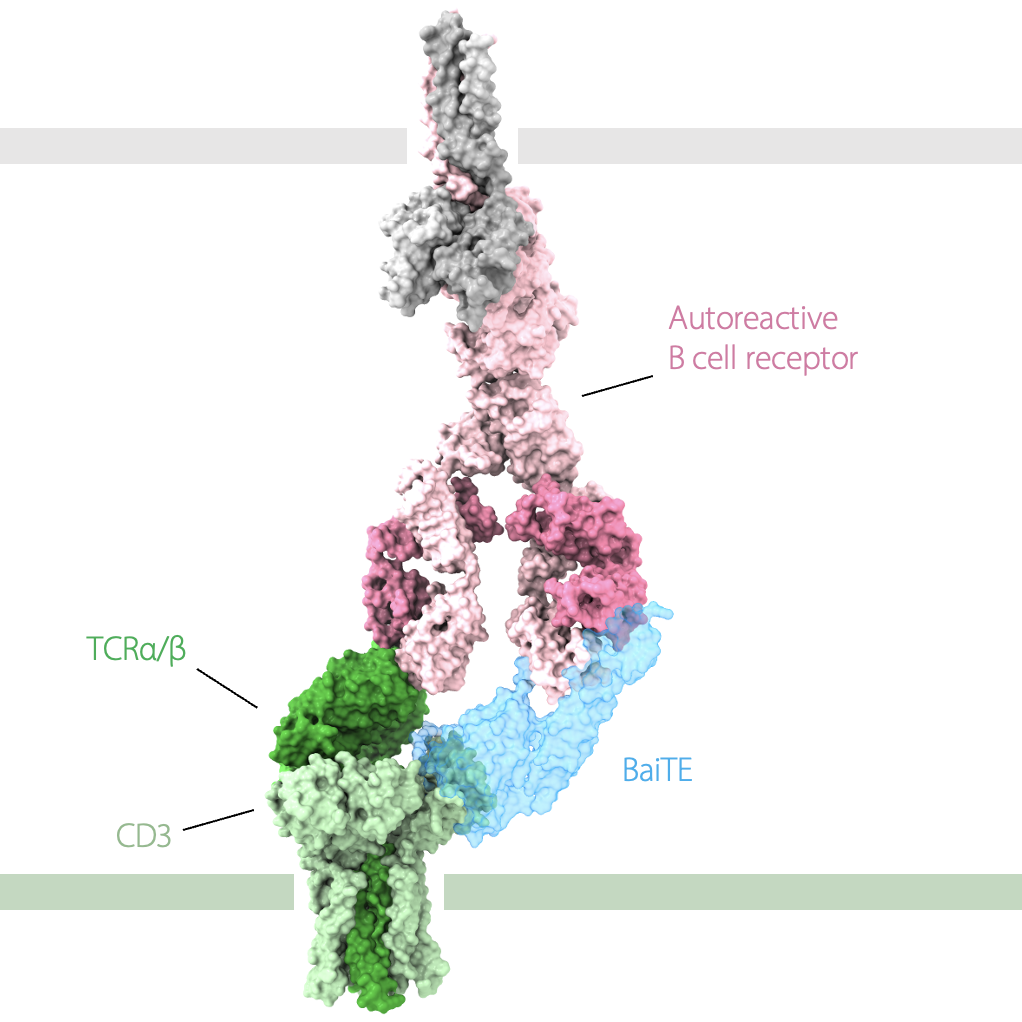
Chimeric antigen receptor (CAR)-T cell therapies targeting B cells have revolutionized the treatment of blood cancers over the past decade and can be curative. These emerging therapies can also achieve drug-free disease remission in patients with severe autoimmune and rheumatic diseases. However, CAR-T cell therapies indiscriminately deplete entire B cell compartments, leaving patients at risk of infectious complications.
Our lab is developing multiple technologies that can overcome this limitation, pairing the unprecedented potency of T cell therapies with therapeutic precision:
Collaborators
Bert Vogelstein, M.D.; Ludwig Center for Cancer Genetics and Therapeutics
Kenneth Kinzler, Ph.D.; Ludwig Center for Cancer Genetics and Therapeutics
Shibin Zhou, M.D., Ph.D.; Ludwig Center for Cancer Genetics and Therapeutics
Suman Paul, M.B.B.S., Ph.D.; Ludwig Center for Cancer Genetics and Therapeutics
Nickolas Papadopoulos, Ph.D.; Ludwig Center for Cancer Genetics and Therapeutics
Chetan Bettegowda, M.D., Ph.D.; Ludwig Center for Cancer Genetics and Therapeutics
Drew Pardoll, M.D., Ph.D.; Bloomberg~Kimmel Institute for Cancer Immunotherapy
Michelle Petri, M.D., M.P.H.; Division of Rheumatology, The Johns Hopkins University
Antony Rosen, M.B.Ch.B., M.S.; Division of Rheumatology, The Johns Hopkins University
Recent Publications
Ma J, Zhu Y, Kong J, Yu D, Toh WH, Jain M, Ni Q, Ge Z, Lin J, Choy J, Cheng L, Konstantopoulos K, Konig MF, Sun SX, Mao HQ. Tuning extracellular fluid viscosity to enhance transfection efficiency. Nat Chem Eng. 2024;1(576–587). https://doi.org/10.1038/s44286-024-00116-3.
Mog BJ, Marcou N, DiNapoli SR, Pearlman AH, Nichakawade TD, Hwang MS, Douglass J, Hsiue EH, Glavaris S, Wright KM, Konig MF, Paul S, Wyhs N, Ge J, Miller MS, Azurmendi P, Watson E, Pardoll DM, Gabelli SB, Bettegowda C, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S. Preclinical studies show that Co-STARs combine the advantages of chimeric antigen and T cell receptors for the treatment of tumors with low antigen densities. Sci Transl Med. 2024 Jul 10;16(755):eadg7123. doi: 10.1126/scitranslmed.adg7123.
Falde SD, Fussner LA, Tazelaar HD, O'Brien EK, Lamprecht P, Konig MF, Specks U. Proteinase 3-specific antineutrophil cytoplasmic antibody-associated vasculitis. Lancet Rheumatol. 2024 Mar 28:S2665-9913(24)00035-3. doi: 10.1016/S2665-9913(24)00035-3.
Nichakawade TD, Ge J, Mog BJ, Lee BS, Pearlman AH, Hwang MS, DiNapoli SR, Wyhs N, Marcou N, Glavaris S, Konig MF, Gabelli SB, Watson E, Sterling C, Wagner-Johnston N, Rozati S, Swinnen L, Fuchs E, Pardoll DM, Gabrielson K, Papadopoulos N, Bettegowda C, Kinzler KW, Zhou S, Sur S, Vogelstein B, Paul S. TRBC1-targeting antibody-drug conjugates for the treatment of T cell cancers. Nature. 2024 Mar 27. doi: 10.1038/s41586-024-07233-2.
Konig MF. The rise of precision cellular therapies. Nat Rev Rheumatol. 2024 Jan 8;. doi: 10.1038/s41584-023-01073-6.
Mog B, Shaw E, Hwang M, Pearlman A, DiNapoli S, Paul S, Bettegowda C, Papadopoulos N, Gabelli S, Petri M, Rosen A, Zhou S, Kinzler K, Vogelstein B, Konig MF. Chimeric Autoantigen-T Cell Receptor (CATCR)-T Cell Therapies to Selectively Target Autoreactive B Cells [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). [presented in Plenary Session III, American College of Rheumatology Convergence 2022]
Zou RS, Liu Y, Gaido OER, Konig MF, Mog BJ, Shen LL, Aviles-Vazquez F, Marin-Gonzalez A, Ha T. Improving the sensitivity of in vivo CRISPR off-target detection with DISCOVER-Seq. Nat Methods. 2023 Apr 6. doi: 10.1038/s41592-023-01840-z. PMID: 37024653.
Konig MF*, Paul S. More on CD19-CAR T Cells in Systemic Lupus Erythematosus. N Engl J Med. 2021 Nov 4;385(19):e67.
Hwang MS, Miller MS, Thirawatananond P, Douglass J, Wright KM, Hsiue EH, Mog BJ, Aytenfisu TY, Murphy MB, Aitana Azurmendi P, Skora AD, Pearlman AH, Paul S, DiNapoli SR, Konig MF, Bettegowda C, Pardoll DM, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S, Gabelli SB. Structural engineering of chimeric antigen receptors targeting HLA-restricted neoantigens. Nat Commun. 2021 Sep 6;12(1):5271.
Pearlman AH, Hwang M, Konig MF, Hsiue E, Douglass J, DiNapoli SR, Mog BJ, Bettegowda C, Pardoll DM, Gabelli SB, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S. Targeting public neoantigens for cancer immunotherapy. Nature Cancer. 2021 May; 2(5):487-497.
Hwang MS, Mog BJ, Douglass J, Pearlman AH, Hsiue EH, Paul S, DiNapoli SR, Konig MF, Pardoll DM, Gabelli SB, Bettegowda C, Papadopoulos N, Vogelstein B, Zhou S, Kinzler KW. Targeting loss of heterozygosity for cancer-specific immunotherapy. Proc Natl Acad Sci U S A. 2021 Mar 23;118(12).
Paul S, Pearlman AH, Douglass J, Mog BJ, Hsiue EH, Hwang MS, DiNapoli SR, Konig MF, Brown PA, Wright KM, Sur S, Gabelli SB, Li Y, Ghiaur G, Pardoll DM, Papadopoulos N, Bettegowda C, Kinzler KW, Zhou S, Vogelstein B. TCR β chain-directed bispecific antibodies for the treatment of T cell cancers. Sci Transl Med. 2021 Mar 10;13(584):eabd3595.
Hsiue EH, Wright KM, Douglass J, Hwang MS, Mog BJ, Pearlman AH, Paul S, DiNapoli SR, Konig MF, Wang Q, Schaefer A, Miller MS, Skora AD, Azurmendi PA, Murphy MB, Liu Q, Watson E, Li Y, Pardoll DM, Bettegowda C, Papadopoulos N, Kinzler KW, Vogelstein B, Gabelli SB, Zhou S. Targeting a neoantigen derived from a common TP53 mutation. Science. 2021 Mar 5;371(6533):eabc8697.
Douglass J, Hsiue EH, Mog BJ, Hwang MS, DiNapoli SR, Pearlman AH, Miller MS, Wright KM, Azurmendi PA, Wang Q, Paul S, Schaefer A, Skora AD, Molin MD, Konig MF, Liu Q, Watson E, Li Y, Murphy MB, Pardoll DM, Bettegowda C, Papadopoulos N, Gabelli SB, Kinzler KW, Vogelstein B, Zhou S. Bispecific antibodies targeting mutant RAS neoantigens. Sci Immunol. 2021 Mar 1;6(57).

Help change the way we treat autoimmune disease —
Our research is supported by:
The Jerome L. Greene Foundation
The Cupid Foundation
The Sol Goldman MS Research Program
The Peter and Carmen Lucia Buck Foundation
The Stephen & Renee Bisciotti Foundation
Blackbird Labs
CureAAV
The Lupus Research Alliance
The Rheumatology Research Foundation
Harrington Discovery Institute
National Institutes of Health